Theme: Current Research Advancements and Challenges in the manufacture and production of HIV Vaccines
HIV Vaccines 2016
OMICS International Conferences invites all the participants from all over the world to attend “15th Annual Conference on HIV Vaccines & Therapeutics” which will be held during December 08-09, 2016 in Philadelphia, USA which includes Prompt Keynote presentations, Oral talks, Poster presentations and Exhibitions. The Theme of the conference is "Current Research Advancements and Challenges in the manufacture and production of HIV Vaccines".
HIV Vaccines 2016 hosting presentations from Global Leaders in the field across the Globe and bringing together vaccine stakeholders including academic researchers, public health clinicians, vaccine policy makers, corporate and vaccine manufacturers. There will be opportunities for those selected to present at the meeting to also publish a manuscripts and abstracts from their talk in the Journal of Vaccines and Vaccination. OMICS International organizes 1000+ Conferences every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Track 1: Preventive HIV Vaccines:
Preventive HIV vaccines are designed to protect HIV negative people from becoming infected or getting sick. Although there is currently no vaccine to prevent HIV, researchers are developing and testing potential HIV vaccines. The goal is to develop a vaccine that can protect people from HIV infection, or at least lessen the chance of getting HIV or AIDS should a person be exposed to the virus.
When your body encounters a microorganism, your immune system mounts an attack on the invader. After the microorganism is defeated, your immune system continues to "remember" how to quickly beat the invader should it try to infect you again. A vaccine is designed to resemble a real microorganism. The vaccine trains your immune system to recognize and attack the real microorganism should you ever encounter it. If you've received an effective vaccine, your immune system will "remember" how to quickly attack and defeat a particular microorganism for many years.
Related Conferences:
15th Annual Conference on HIV Vaccines & Therapeutics, December 08-09, 2016 Philadelphia, USA; Vaccines conferences, June 16-18, 2016, Rome, Italy; Annual Conference on H1N1 & Influenza Vaccines, July 25-26, 2016, USA; 12th Global Vaccines & Vaccination Summit and Expo, Oct 20-22, 2016, UAE; 11th Global Summit and Expo on Vaccines & Vaccination, Sept 12-14, 2016, USA; HIV Vaccines (X8), USA; HIV Persistence: Pathogenesis and Eradication (X7), USA; American Conference for the Treatment of HIV, USA; The 20th International AIDS Conference, Australia; 2015 National HIV Prevention Conference, USA
Track 2: Therapeutic HIV vaccines:
A therapeutic HIV vaccine (also known as a treatment vaccine) is a vaccine used in the treatment of an HIV infected person. Therapeutic HIV vaccines are designed to boost the body's immune response to HIV in order to better control the infection.
Currently, there are no therapeutic HIV vaccines approved by the Food and Drug Administration (FDA). However, therapeutic HIV vaccines are being tested in clinical trials to find out if they are safe and effective in treating people with HIV. Researchers hope that if therapeutic vaccines are able to strengthen the body's natural anti-HIV immune response, people with HIV will not have to rely exclusively on the antiretroviral drugs now used to treat HIV infection. Currently, anti-retroviral drugs must be taken for life, and some cause serious side effects.
All experimental therapeutic HIV vaccines are in very early stages of research, and no therapeutic vaccine is anticipated to be available to the general public for many years, if at all.
Related Conferences:
11th Global Summit and Expo on Vaccines & Vaccination, Sept 12-14, 2016, USA; Annual Conference on HIV Vaccines & Therapeutics, December 08-09, 2016 Philadelphia, USA; Vaccines conferences, June 16-18, 2016, Rome, Italy; Annual Conference on H1N1 & Influenza Vaccines, July 25-26, 2016, USA; 12th Global Vaccines & Vaccination Summit and Expo, Oct 20-22, 2016, UAE; HIV Vaccines (X8), USA; HIV Persistence: Pathogenesis and Eradication (X7), USA; American Conference for the Treatment of HIV, USA; The 20th International AIDS Conference, Australia; 2015 National HIV Prevention Conference, USA
Track 3: Subunit vaccines:
A subunit vaccine presents an antigen to the immune system without introducing viral particles, whole or otherwise. One method of production involves isolation of a specific protein from a virus and administering this by itself.
Instead of the entire microbe, subunit vaccines include only the antigens that best stimulate the immune system. In some cases, these vaccines use epitopes—the very specific parts of the antigen that antibodies or T cells recognize and bind to. Because subunit vaccines contain only the essential antigens and not all the other molecules that make up the microbe, the chances of adverse drug reactions to the vaccine are lower.
Subunit vaccines can contain anywhere from 1 to 20 or more antigens. Of course, identifying which antigens best stimulate the immune system is a tricky, time-consuming process. Once scientists do that, however, they can make subunit vaccines in one of two ways:
- They can grow the microbes in the laboratory and then use chemicals to break it apart and gather the important antigens.
- They can manufacture the antigen molecules from the microbe using recombinant DNA technology. Vaccines produced this way are called “recombinant subunit vaccines.”
A recombinant subunit vaccine has been made for the hepatitis B virus. Scientists inserted hepatitis B genes that code for important antigens into common baker’s yeast. The yeast then produced the antigens, which the scientists collected and purified for use in the vaccine. Research is continuing on a recombinant subunit vaccine against hepatitis C virus.
Related Conferences:
Vaccines conferences, June 16-18, 2016, Rome, Italy; Annual Conference on HIV Vaccines & Therapeutics, December 08-09, 2016 Philadelphia, USA; Annual Conference on H1N1 & Influenza Vaccines, July 25-26, 2016, USA; 12th Global Vaccines & Vaccination Summit and Expo, Oct 20-22, 2016, UAE; 11th Global Summit and Expo on Vaccines & Vaccination, Sept 12-14, 2016, USA; HIV Vaccines (X8), USA; HIV Persistence: Pathogenesis and Eradication (X7), USA; American Conference for the Treatment of HIV, USA; The 20th International AIDS Conference, Australia; 2015 National HIV Prevention Conference, USA
Track 4: Recombinant vector vaccines:
Recombinant vector vaccines are experimental vaccines similar to DNA vaccines, but they use an attenuated virus or bacterium to introduce microbial DNA to cells of the body. “Vector” refers to the virus or bacterium used as the carrier.
In nature, viruses latch on to cells and inject their genetic material into them. In the lab, scientists have taken advantage of this process. They have figured out how to take the roomy genomes of certain harmless or attenuated viruses and insert portions of the genetic material from other microbes into them. The carrier viruses then ferry that microbial DNA to cells. Recombinant vector vaccines closely mimic a natural infection and therefore do a good job of stimulating the immune system.
Attenuated bacteria also can be used as vectors. In this case, the inserted genetic material causes the bacteria to display the antigens of other microbes on its surface. In effect, the harmless bacterium mimics a harmful microbe, provoking an immune response.
Researchers are working on both bacterial and viral-based recombinant vector vaccines for HIV, rabies, and measles.
Relevant Conferences:
4th HIV/AIDS, STDs, & STIs Conference, November 30-December 02, 2015, USA; 2nd Infectious Diseases Conference, August 25-27, 2016, USA; 2nd Influenza Conference, September 12-14, 2016, Germany; Infection Prevention and Control Conference, Aug 1-2, 2016, Germany; Annual Conference on HIV Vaccines, December 08-09, 2016 Philadelphia, USA; World STI & HIV 2015 Congress, Australia; 19th Annual United States Conference on AIDS, USA; 10th International Workshop on HIV Transmission – Principles of Intervention, USA; 5th Workshop on HCV Therapy Advances – New Antivirals in Clinical Practice, Netherlands; 18th Bangkok International Symposium on HIV Medicine, Thailand
Track 5: DNA vaccines:
DNA vaccination is a technique for protecting an animal against disease by injecting it with genetically engineered DNA so cells directly produce an antigen, resulting in a protective immunological response.
Still in the experimental stages, these vaccines show great promise, and several types are being tested in humans. DNA vaccines take immunization to a new technological level. These vaccines dispense with both the whole organism and its parts and get right down to the essentials: the microbe’s genetic material. In particular, DNA vaccines use the genes that code for those all-important antigens.
Researchers have found that when the genes for a microbe’s antigens are introduced into the body, some cells will take up that DNA. The DNA then instructs those cells to make the antigen molecules. The cells secrete the antigens and display them on their surfaces. In other words, the body’s own cells become vaccine-making factories, creating the antigens necessary to stimulate the immune system.
A DNA vaccine against a microbe would evoke a strong antibody response to the free-floating antigen secreted by cells, and the vaccine also would stimulate a strong cellular response against the microbial antigens displayed on cell surfaces. The DNA vaccine couldn’t cause the disease because it wouldn’t contain the microbe, just copies of a few of its genes. In addition, DNA vaccines are relatively easy and inexpensive to design and produce.
Relevant Conferences:
12th Global Vaccines & Vaccination Summit and Expo, Oct 20-22, 2016, UAE; Vaccines conferences, June 16-18, 2016, Rome, Italy; 15th Annual Conference on HIV Vaccines, December 08-09, 2016 Philadelphia, USA; Annual Conference on H1N1 & Influenza Vaccines, July 25-26, 2016, USA; 11th Global Summit and Expo on Vaccines & Vaccination, Sept 12-14, 2016, USA; 18th Bangkok International Symposium on HIV Medicine, Thailand; HIV Persistence: Pathogenesis and Eradication (X7), USA; American Conference for the Treatment of HIV, USA; The 20th International AIDS Conference, Australia; 2015 National HIV Prevention Conference, USA
Track 6: HIV Vaccines Trials:
Vaccines are evaluated through a series of clearly defined controlled studies to assess these investigational products for safety, immunogenicity and efficacy before they are approved for licensure. All clinical vaccine trials are bound by international ethical guidelines and, in the case of US trials, Food and Drug Administration (FDA) regulations.
Good Clinical Practice is defined by the International Conferences on Harmonization (ICH) as: “A standard for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials that provides assurance that the data and reported results are credible and accurate, and that the rights, integrity and confidentiality of trial subjects are protected.”
Historically, vaccines have been our best weapon against the world’s deadliest infectious diseases, including smallpox, polio, measles, and yellow fever. Unfortunately, we do not have a vaccine for HIV. The virus has unique ways of evading the immune system, and the human body seems incapable of mounting an effective immune response against it. As a result, scientists do not have a clear picture of what is needed to provide protection against HIV.
Relevant Conferences:
12th Global Vaccines & Vaccination Summit and Expo, Oct 20-22, 2016, UAE; Vaccines conferences, June 16-18, 2016, Rome, Italy; 15th Annual Conference on HIV Vaccines, December 08-09, 2016 Philadelphia, USA; Annual Conference on H1N1 & Influenza Vaccines, July 25-26, 2016, USA; 11th Global Summit and Expo on Vaccines & Vaccination, Sept 12-14, 2016, USA; 18th Bangkok International Symposium on HIV Medicine, Thailand; HIV Persistence: HIV Vaccines (X8), USA; American Conference for the Treatment of HIV, USA; The 20th International AIDS Conference, Australia; 2015 National HIV Prevention Conference, USA
Track 7: Unwanted effects of HIV Vaccines:
If you have HIV (human immunodeficiency virus) or AIDS (acquired immunodeficiency syndrome), you should take special precautions against other infections, such as the flu. That's because you have a disease that makes it difficult for your immune system to fight them. Vaccines (immunizations) can help your body defend itself against infections. However, if you have HIV/AIDS immunizations may effect you differently than people who don't have HIV/AIDS.
Not all vaccines are safe for people with HIV/AIDS. Vaccines made from live viruses should be avoided because they may cause a mild case of the disease. Live vaccines are a weaker form of the germ that causes a particular disease. People with HIV/AIDS should receive vaccines made from inactivated diseases. Inactivated vaccines don't contain a living germ.
Vaccine Side Effects and HIV/AIDS:
Anyone, regardless of their HIV status, is at risk of side effects associated with vaccines, including:
- Pain, redness, or swelling at the place where you receive the shot
- Weakness
- Fatigue
- Nausea
However, if you have HIV/AIDS, vaccines carry additional risks including:
- Vaccines may increase your viral load
- Vaccines may not work as well if your CD4 count is very low. CD4 cells are a type of immune cell. It may help to take strong antiretroviral medications before having a vaccine if your CD4 count is low.
- Vaccines made from a live virus may cause you to get the disease the vaccine is supposed to prevent.
Relevant Conferences:
15th Annual Conference on HIV Vaccines, December 08-09, 2016 Philadelphia, USA; Vaccines conferences, June 16-18, 2016, Rome, Italy; Annual Conference on H1N1 & Influenza Vaccines, July 25-26, 2016, USA; 12th Global Vaccines & Vaccination Summit and Expo, Oct 20-22, 2016, UAE; 11th Global Summit and Expo on Vaccines & Vaccination, Sept 12-14, 2016, USA; HIV Vaccines (X8), USA; HIV Persistence: Pathogenesis and Eradication (X7), USA; American Conference for the Treatment of HIV, USA; The 20th International AIDS Conference, Australia; 2015 National HIV Prevention Conference, USA
Track 8: Vaccine Administration:
The administration of antigenic material (a vaccine) to stimulate an individual's immune system to develop adaptive immunity to a pathogen. Vaccines can prevent or ameliorate morbidity from infection.
There are two basic types of vaccines: live attenuated and inactivated. The characteristics of live and inactivated vaccines are different, and these characteristics determine how the vaccine is used.
Live attenuated vaccines are produced by modifying a disease-producing (“wild”) virus or bacteria in a laboratory. The resulting vaccine organism retains the ability to replicate (grow) and produce immunity, but usually does not cause illness. Live attenuated vaccines include live viruses and live bacteria.
Inactivated vaccines can be composed of either whole viruses or bacteria, or fractions of either:
- Fractional vaccines are either protein-based or polysaccharide-based.
- Protein-based vaccines include toxoids (inactivated bacterial toxin), and subunit or subvirion products.
- Most polysaccharide-based vaccines are composed of pure cell-wall polysaccharides from bacteria.
- Conjugate polysaccharide vaccines are those in which the polysaccharide is chemically linked to a protein. This linkage makes the polysaccharide a more potent vaccine.
Relevant Conferences:
15th Annual Conference on HIV Vaccines, December 08-09, 2016 Philadelphia, USA; Vaccines conferences, June 16-18, 2016, Rome, Italy; Annual Conference on H1N1 & Influenza Vaccines, July 25-26, 2016, USA; 12th Global Vaccines & Vaccination Summit and Expo, Oct 20-22, 2016, UAE; 11th Global Summit and Expo on Vaccines & Vaccination, Sept 12-14, 2016, USA; The 20th International AIDS Conference, Australia; 2015 National HIV Prevention Conference, USA; 34th Annual Infectious Diseases Conference, USA; XXV International HIV Drug Resistance Workshop, USA; 23rd Conference on Retro-viruses and Opportunistic Infections, USA
Track 9: HIV Vaccines Research:
HIV is a very complex, highly changeable virus, which makes speedy development of successful preventive HIV vaccines very difficult, but not impossible. It also takes many years to conduct the research, including the careful clinical testing that will lead to a safe and effective vaccine.
Researchers from around the world have been working for more than two decades to create a vaccine that will protect people against HIV infection. NIAID supports the HIV Vaccine Trials Network (HVTN), an international collaboration of scientists and educators searching for an effective and safe HIV vaccine. The U.S. Military HIV Research Program (MHRP) is also engaged in HIV vaccine research and led a large collaboration of clinical scientists also funded by NIAID in implementing a vaccine clinical trial that showed for the first time that an HIV vaccine is possible.
Scientists are continuing to create and test HIV vaccines—in the lab, in animals, and even in human subjects. These vaccine trials help researchers to learn whether a vaccine will work and if it can be safely given to people.
Relevant Conferences:
15th Annual Conference on HIV Vaccines, December 08-09, 2016 Philadelphia, USA; Vaccines conferences, June 16-18, 2016, Rome, Italy; Annual Conference on H1N1 & Influenza Vaccines, July 25-26, 2016, USA; 12th Global Vaccines & Vaccination Summit and Expo, Oct 20-22, 2016, UAE; 11th Global Summit and Expo on Vaccines & Vaccination, Sept 12-14, 2016, USA; IV Persistence: Pathogenesis and Eradication, USA; American Conference for the Treatment of HIV, USA; 17th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy, USA; 14th European Meeting on HIV & Hepatitis Treatment Strategies & Antiviral Drug Resistance- Treatment Strategies & Antiviral Drug Resistance, Italy
15th Annual Conference on HIV Vaccines & Therapeutics December 08-09, 2016 Philadelphia, USA
OMICS International Conferences invites all the participants from all over the world to attend "15th Annual Conference on HIV Vaccines & Therapeutics" during December 08-09, 2016 Philadelphia, USA which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions.
Following the great success of the 5th Asia Pacific Global Summit and Expo on Vaccines & Vaccination, July 27-28, 2015 Brisbane, Australia, OMICS Group International is organizing the 15th Annual Conference on HIV Vaccines & Therapeatics which is going to be held in Philadelphia, USA in 2016. This will be the latest in the OMICS group annual vaccine meeting series expecting with 250 Plus Attendees, to provide a forum where key vaccine stakeholders including academic researchers, public health clinicians, veterinarians, vaccine policy makers, and manufacturers can exchange ideas and collaborate. There will be opportunities for those chosen to present at the meeting to publish a manuscript based on their presentation in the Journal of Vaccines & Vaccination or its sister publication, Immunome Research. OMICS International organizes a conference series of 1000+ Global Events inclusive of 300+ Conferences, 500+ Upcoming and Previous Symposiums and Workshops in USA, Europe & Asia with support from 1000 more scientific societies and publishes 700+ Open access journals which contains over 30000 eminent personalities, reputed scientists as editorial board members.
Why to attend???
With members from around the world focused on learning about Vaccinology/Immunology and its advances; this is your best opportunity to reach the largest assemblage of participants from the Immunology/Vaccinology community. Conduct presentations, distribute information, meet with current and potential scientists, make a splash with new vaccines developments, and receive name recognition at this event. World-renowned speakers, the most recent techniques, developments, and the newest updates in HIV-Immunology/Vaccinology are hallmarks of this conference.
Target Audience:
- Vaccinology/Immunology Students, Scientists
- Vaccinology/Immunology Researchers
- Vaccinology/Immunology Faculty
- Medical Colleges
- Vaccinology/Immunology Associations and Societies
- Business Entrepreneurs
- Training Institutes
- Software developing companies
- Manufacturing Medical Devices Companies
- Data Management Companies
- Machinery Manufacturing Companies
- Others who are related
The 15th Annual Conference on HIV Vaccines & Therapeutics to be held at Philadelphia, USA during December 08-09, 2016 hosted by OMICS Group Conferences through the theme "Current Research Advancements and Challenges in the manufacture and production of HIV Vaccines", conference will explore the advances in Vaccines and vaccination. This conference could be an exceptional event that brings along a novel and International mixture of giant and medium cognizance on vaccines and vaccination, leading universities engendering the conference an ideal platform to apportion expertise, foster collaborations across trade and world, and assess elevating technologies across the world. OMICS International Publishes 400+ Open access journals which contains over 30000 eminent personalities, reputed scientists as editorial board members and Organizes 300+ Conferences every year across USA, Europe & Asia with support from 1000 more scientific societies.
Vaccination is a key to eradicate the diseases. It is a subject for the professionals who looks beyond the clinical prospects. Vaccines Asia Pacific-2016 amasses all the extroverts under one roof of current advances.
For more details visit- http://hiv.conferenceseries.com/
Vaccinology is a major field in eradication of diseases majorly Hiv vaccines need to develop. Traditional approaches to vaccine design were to emasculate or inactivate the human pathogen or a cognate animal homologue, Vaccinology is the science of vaccine development and how the immune system responds to vaccines, but additionally includes perpetual evaluation of immunization programs and vaccine safety and efficacy, as well as surveillance of the epidemiology of vaccine-preventable diseases. This chapter provides a brief overview of some of the main concepts of immunology and vaccinology as they relate to immunization.
Why USA-Philadelphia?
In 2015 there are 23 vaccines and related conferences are held in USA. From thar three are from Philadelphia
Philadelphia City Council provides a free immunisation service for children to help protect them against childhood diseases such as measles, whooping cough, polio and tetanus. Providing a free immunisation service is another way Council delivers towards the Atlanta Vision. Around 22 Biological giants are working in USA. Around 15 top universities are working on vaccines research in USA.
Why to attend???
HIV Vaccines-2016 could be an event that brings along a novel and International mixture of giant and medium Vaccinology innovation and incipient trends in vaccines and vaccination, leading universities and vaccinology, vaccination research analysis establishments creating the conference an ideal platform to share expertise, foster collaborations across trade and world, and assess rising technologies across the world. World-renowned speakers, the most recent techniques, tactics, and the newest updates in Vaccinology research are hallmarks of this conference.
A Unique Opportunity for Advertisers and Sponsors at this International event:
http://hiv.conferenceseries.com/
Major Vaccines Associations in & around Miami.
· Vaccine research Association
· US vaccination association
· USA medical association
· US veterinary association
· Association of veterinary vaccination
Major Vaccines Associations Globally
· The International Society for Interferon and Cytokine Research
· The International Society of Neuroimmunology
· The International Union of Immunological Societies
· The Society for Mucosal Immunology
· The Society for Leukocyte Biology
· Transplantation Society
· British Society for Immunology
· Cell Death Society
· European Federation of Immunological Societies
· European Society of Gene Therapy
· Federation of African Immunological Societies
· Federation of American Societies for Experimental Biology
· Federation of Clinical Immunology Societies
· Immune Deficiency Foundation
· Infectious Diseases Society of America
Target Audience:
HIV Vaccines-2016 Targets, CEO’s, Directors, Scientists, Professors, Students of all Biotech & Pharma companies, Universities and colleges globally.
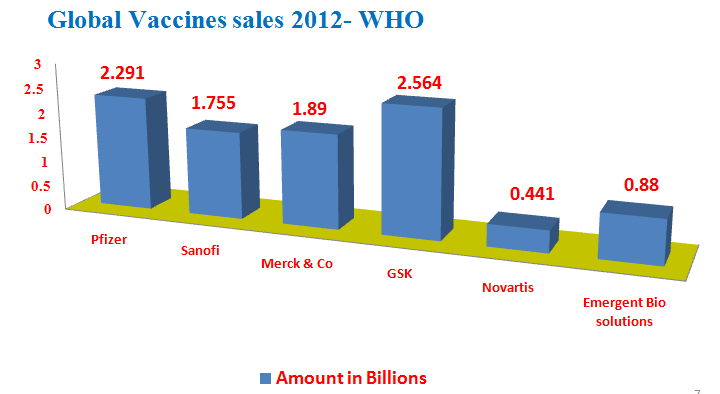
Vaccines sales in global leader companies 2012(1st quarter):
· Pfizer 2.2 B $
· Sanofi 1.7 B $
· Merck & Co 1.8 B $
· GSK 2.5 B $
· Novartis 0.44 B $
· EBS 0.88 B $
Companies Associated with Vaccines
· CareDx
· Atara
· Biotherapeutics
· Benitec Limited
· InterMune
· Biotron Limited
· BioDiem
· Antisense Therapeutics Limited
· GlaxoSmithKline USA Pty Ltd
· Bionomics
· USA Pharmaceutical Industries (API)
· BioPharmica Limited
Market Analysis of Vaccines
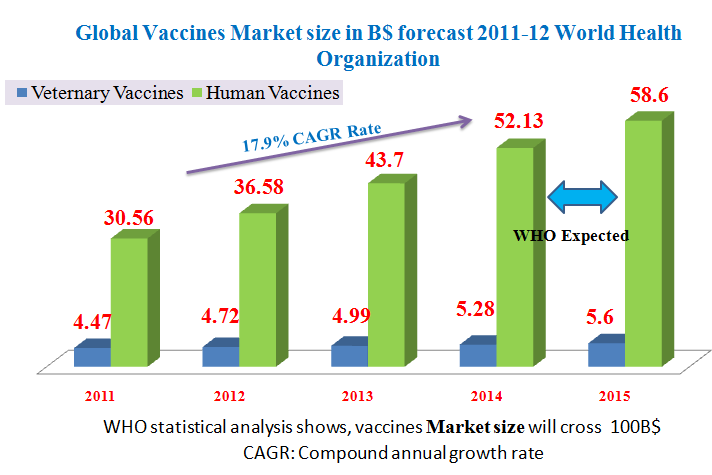
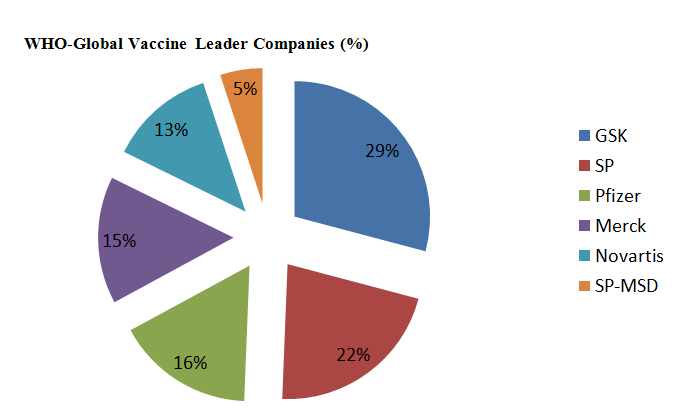
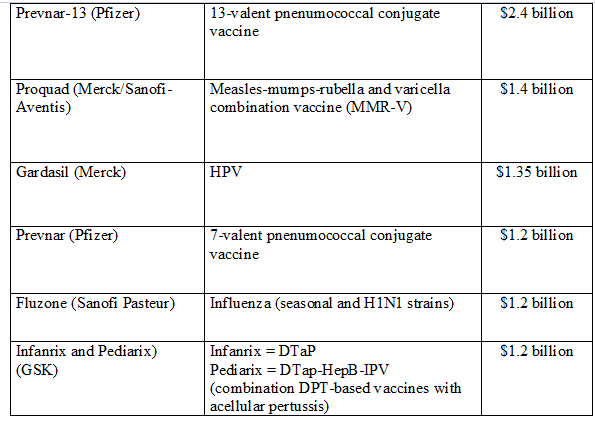
Tripled in value from USD 5B in 2000 to almost USD 24 B in 2013, Global market projected to rise to USD 100 B by 2025. More than 120 new products in the development pipeline. 60 are of importance for developing countries. UNICEF had a market of 1,03 B$ in 2011, PAHO had 400 million dollars in 2011 and total UN market of 340 million dollars in 2002, 1,430 billion dollars in 2011.
Overall Statasticks
Conference Highlights
- Preventive HIV Vaccines
- Therapeutic HIV vaccines
- Subunit vaccines
- Recombinant vector vaccines
- Unwanted effects of HIV Vaccines
- HIV Vaccines Research
- Stages of HIV infection
- Specific Vaccines on Hiv Subjects/Patients
- Immunizations and HIV
- Hiv vaccine development
- STDs in Hiv Patients
- Hepatitis & HIV
- Safety of Vaccinations
- Bacterial Infections in Hiv Patients
- Fungal Infections in Hiv Patients
- Malignancies (Cancers) in Hiv Patients
- Viral Infections In Hiv Patients
- Protozoal Infections In Hiv Patients
- Neurological Conditions In Hiv Patients
- Causes of HIV
- Transmission of HIV
- HIV testing
- HIV Vaccine
- HIV Vaccine Trials
- AIDS Vaccine
- HIV Saliva
- HIV Prevention
- Infectious Diseases
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | December 08-09, 2016 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
Abstracts will be provided with Digital Object Identifier by